![]()
![]()
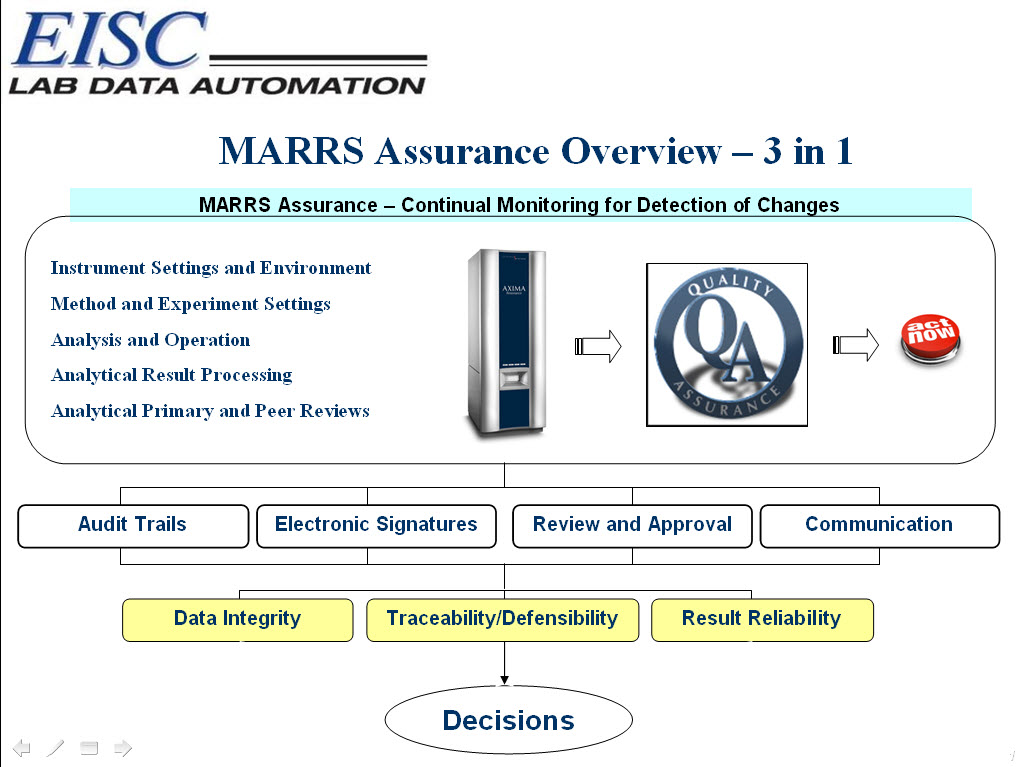
Networking 21 CFR Part 11 compliance through the MARRS™ Assurance for automated communication of primary and secondary review. From technician to peer review with complete data quality assurance and traceability, all within seconds.
For more information on how EISC is providing data automation solutions for labs just like yours, and to schedule a live internet demo
and begin now to experience the ease of lab data automation.
0